
Draw the electron dot structure of chlorine molecule Brainly.in
Please enable Javascript in order to use PubChem website. Chlorine | Cl2 | CID 24526 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
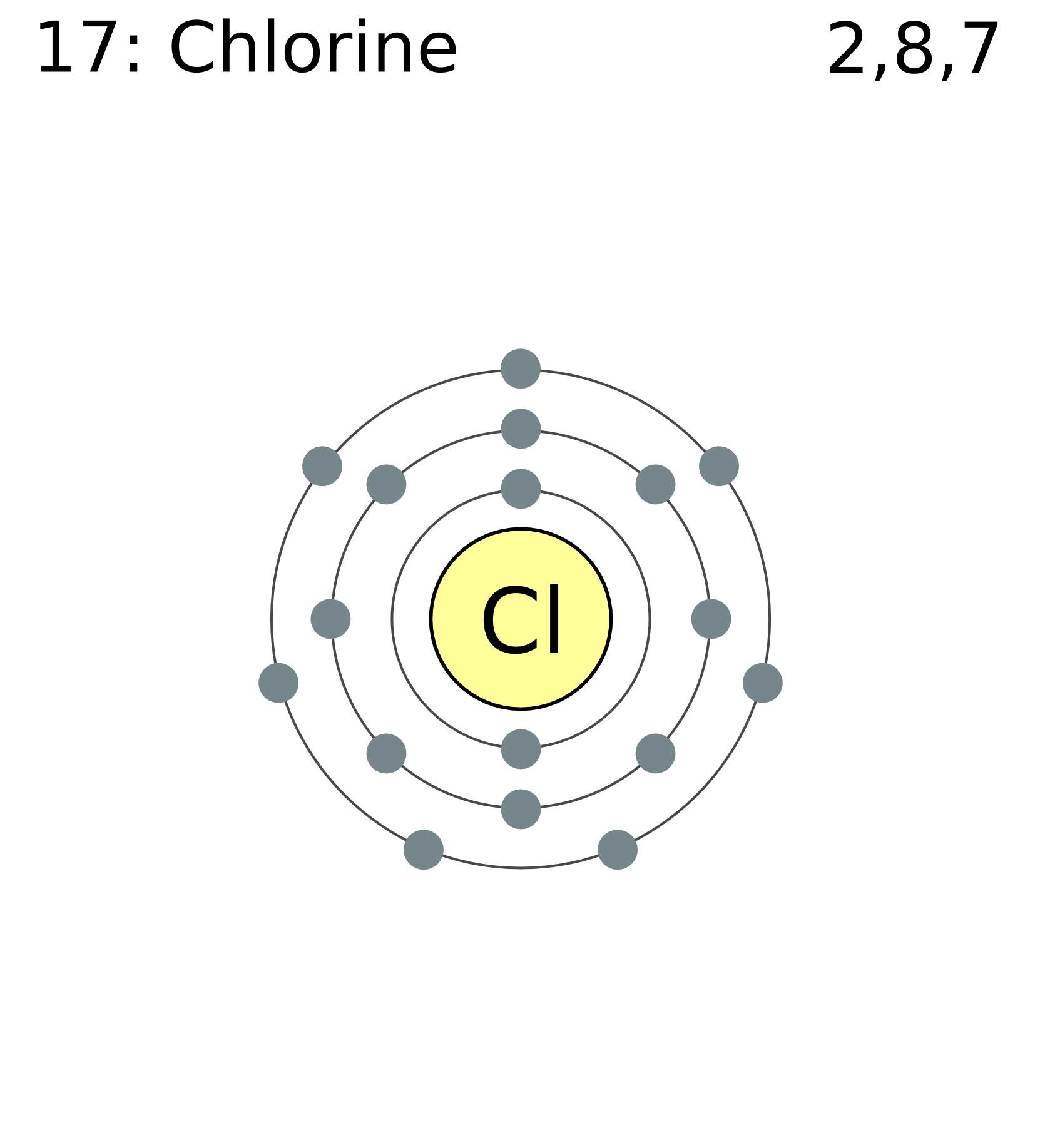
FileElectron shell 017 chlorine.png Wikimedia Commons
Chlorine is a diatomic molecule and contains only two chlorine atoms. Lewis structure of chlorine molecule contains only one Cl-Cl bond and each chlorine atom has three lone pairs. It is very easy to draw the Cl 2 lewis structure. Cl 2 lewis structure There is only a single bond between chlorine atoms and three lone pairs on each chlorine atoms.

Chlorine Pentafluoride Lewis / Chlorine pentafluoride is a square pyramidal molecule. Ajor Png
Atomic Structure. Why does a Chlorine Molecule have a Covalent Bond?. Chlorine is a non-metal. A chlorine atom has 7 electrons in its outer shell. Chlorine is in group 7 of the periodic table. Two chlorine atoms will each share one electron to get a full outer shell and form a stable Cl 2 molecule.. This is a picture of the shared electrons making a covalent bond in a chlorine molecule.
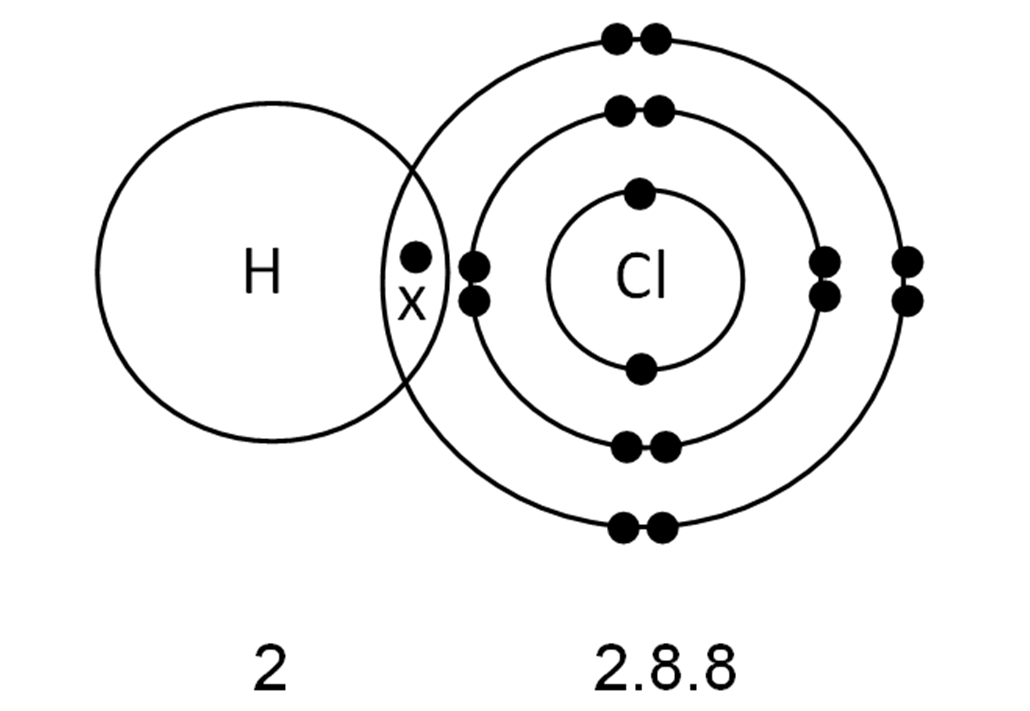
Chem Easy Formation of covalent bond in chlorine molecule
Formula: Cl 2 Molecular weight: 70.906 IUPAC Standard InChI: InChI=1S/Cl2/c1-2 IUPAC Standard InChIKey: KZBUYRJDOAKODT-UHFFFAOYSA-N CAS Registry Number: 7782-50-5 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript .

Atom chlorine Royalty Free Vector Image VectorStock
Chlorine forms a series of oxides (Table 10.3. 2) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. The physical properties of the oxides are summarized in Table 10.3. 2. While, the oxides of chlorine are not very stable (in fact several are shock sensitive and are prone to explode) the conjugate oxyacids are stable.

Chlorine Cl (Element 17) of Periodic Table NewtonDesk
Molecular Formula Cl Average mass 70.906 Da Monoisotopic mass 69.937706 Da ChemSpider ID 22933 More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users 7782-50-5 [RN] Bertholite Chloor [Dutch] Chlor [German] [ACD/IUPAC Name]
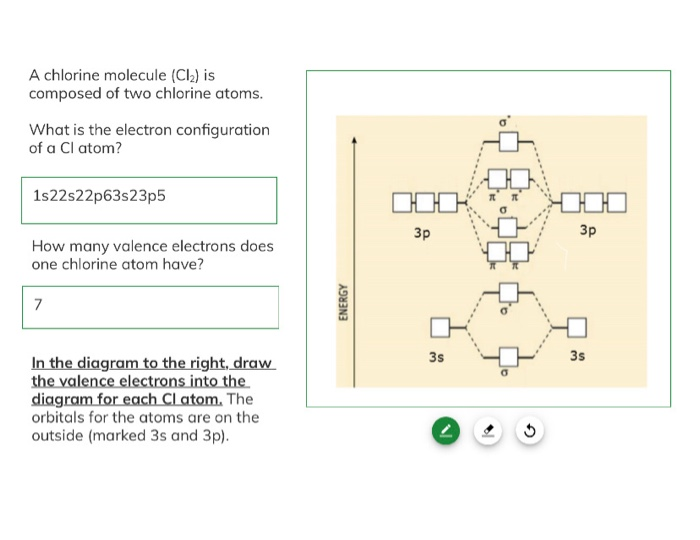
Solved A chlorine molecule (Cl) is composed of two chlorine
1. Determine the total number of valence electrons in the chlorine molecule. There are two chlorine atoms in the chlorine molecule. Each chlorine atom, as a group VIIA element in the periodic table, has seven electrons in its outer shell. Therefore, the total number of valence electrons= 7 (2)= 14. 2.
Chemistry model salt molecule diatomic sodium chlorine NaCl scientific element formula
The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. This allows each halogen atom to have a noble gas electron configuration.. Step 2: Draw a skeleton structure of the molecule, arranging the atoms around a central.

Chlorine Benefits, Chemical Safety of Chlorine, Properties & Facts
Exercise 3.3.4.3 3.3.4. 3. Construct a qualitative molecular orbital diagram for chlorine, Cl 2. Compare the bond order to that seen in the Lewis structure (remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital). Answer.

Aufbau Diagram For Chlorine
1. Count total valence electron in Cl2 For making any lewis diagram our first step is to determine how many valence electron a molecule contain. So, we have to find how many valence electrons available for drawing the Cl2 lewis structure. For this just look at the periodic group of Chlorine in the periodic table.

"Chlorine Molecule" Tshirt by EvasDreams Redbubble
The atomic number of this chemical element is 17. It appears as a pale yellow-green gas. Liquid chlorine can cause skin burn and chlorine in its gaseous form irritates the mucous membrane. Its position as per the periodic table is between fluorine and bromine. Its electronic configuration is [Ne]3s23p5.

Draw the electron dot structure of chlorine molecule Brainly.in
Let us follow some steps to draw the Lewis structure of chlorine dioxide: Step 1: Find the total valence electrons in one molecule of chlorine dioxide. It is 20 as chlorine has 7 valence electrons and oxygen has 6 valence electrons. There are two oxygen molecules in chlorine dioxide so the total is 19.
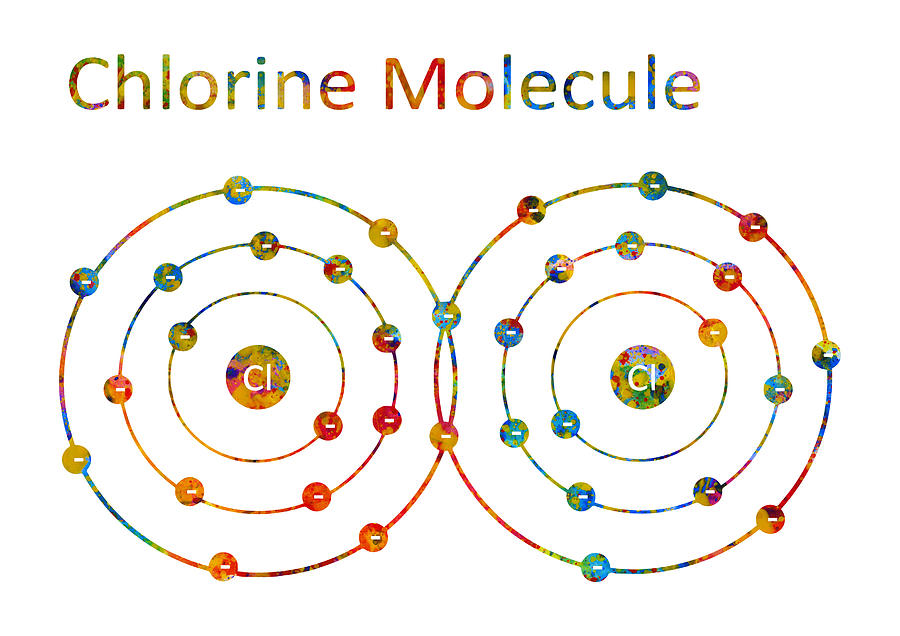
Chlorine Molecule Digital Art by Erzebet S
A step-by-step explanation of how to draw the Cl2 Lewis Dot Structure (Diatomic Chlorine).Note that Diatomic Chlorine is often called Molecular Chlorine or j.
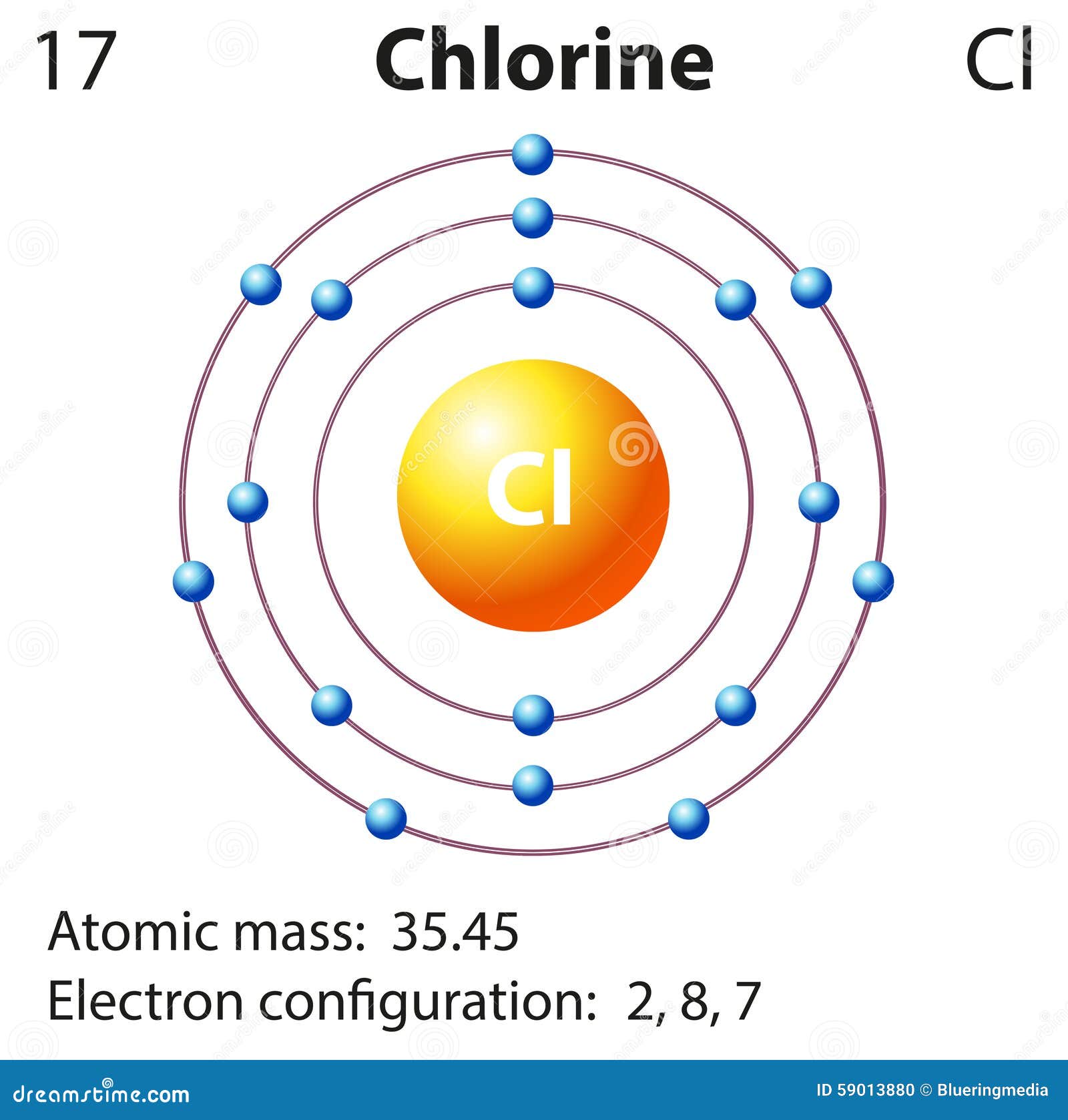
Diagram Representation Of The Element Chlorine Stock Illustration Image 59013880
Chlorine atoms are covalently bonded to form a diatomic Cl 2 molecule. Cl 2 Lewis structure consists of two chlorine atoms linked by a single bond with three lone pairs on each chlorine. Table of Contents How to draw Lewis Structure for Cl 2 Molecular Geometry of Cl 2 Hybridization of Cl 2 Polarity of Cl 2 Frequently Asked Questions - FAQs

A chlorine molecule forms a covalent bond Electronics And Engineering Lab
To write the orbital diagram for the Chlorine atom (Cl) first we need to write the electron configuration for just Cl. To do that we need to find the number.
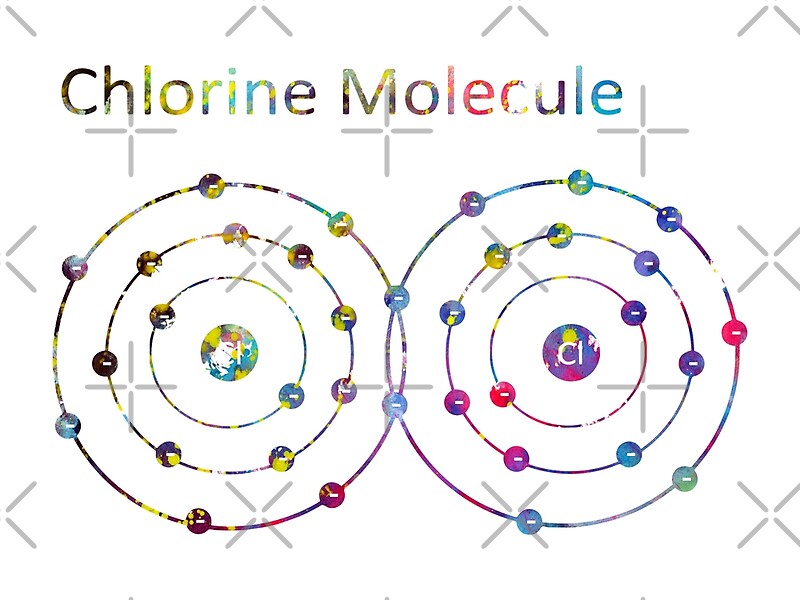
"Chlorine Molecule" by erzebetth Redbubble
A step-by-step explanation of how to draw the Cl2 Lewis Dot Structure (Chlorine gas).For the Cl2 structure use the periodic table to find the total number of.